You have no items in your cart.

Flu cases still high as first human universal vaccine trial begins
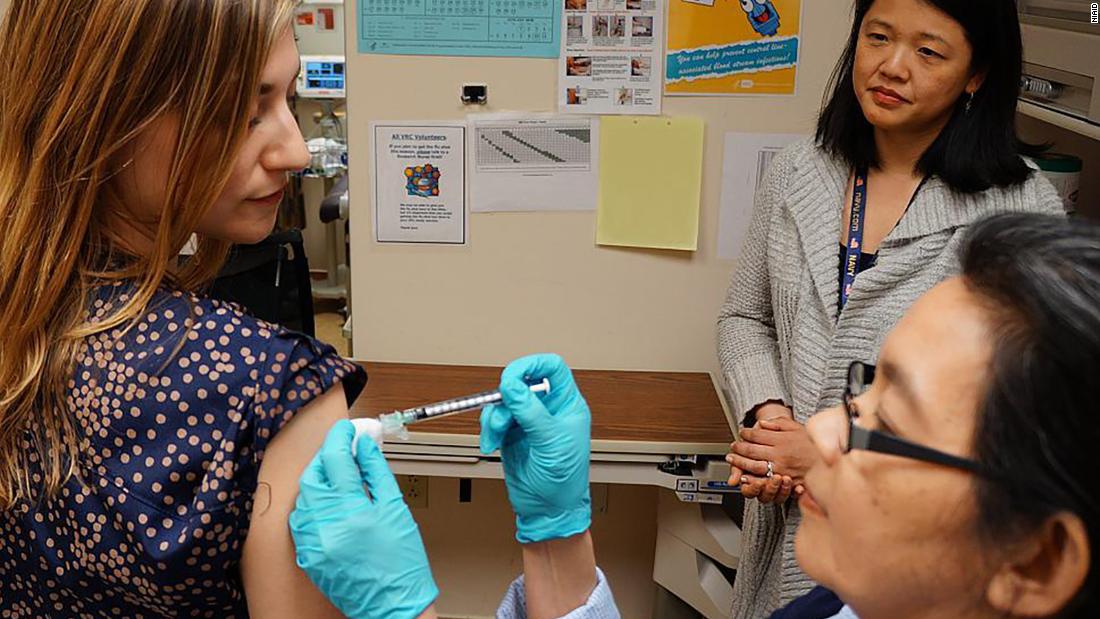
(CNN)Most states are seeing an abnormally high variety of influenza clients as the season ends. The extremely first human experiments with a brand-new universal influenza shot have actually started at the National Institutes of Health.
“Here we remain in April, and we’re still having significant influenza,” stated Dr. William Schaffner, medical director of the National Foundation for Infectious Diseases. “It’s decreased however still considerable.” Throughout the previous week, 34 states and Puerto Rico reported prevalent activity.
Longer than in 2015, less lethal
The total hospitalization rate had to do with 56 for every single 100,000 individuals for the week ended March 30, with the greatest rate, about 182 per 100,000, amongst grownups over 65, the company reported.
The CDC likewise approximated that there have actually depended on 549,000 influenza hospitalizations throughout the 2018-19 season.Meanwhile, reports of flu-like disease are reducing. While 3.8% of individuals who went to a center or medical professional’s workplace suffered flu-like signs the previous week, just 3.2% did so throughout the week ending March 30.
Nationally, H1N1 viral pressures have actually been primary for the season in general. That stated, all areas of the nation have actually been H3N2-predominant for the previous 3 weeks. The H3N2 stress, dominant throughout in 2015’s 2017-18 influenza season, usually triggers even worse influenza signs, causing more deaths and hospitalizations.
“We’ve had the supremacy of H1N1 yield to now a supremacy or a minimum of an equivalence of H3N2, which implies that the pressure that triggers more extreme illness is in fact producing a significant quantity of late season influenza this year,” Schaffner stated.
Finally, the CDC anticipates influenza activity to stay raised for a number of more weeks putting the country on track for a long season.
“We are having a distinct, extended influenza season,” Schaffner stated. He forecasts that the continuing season will bring much more disease, deaths and hospitalizations, “due to the fact that this season is going to go through April; it’s not going to shut off right away. Which’s a late season.”
Experimental vaccine breaks brand-new ground
“It’s tough to consult with you due to the fact that all my toes and fingers are constrained from being crossed,” Schaffner stated, joking about his high expect the brand-new influenza vaccine that got in scientific trials Wednesday.
This speculative vaccine, called H1ssF_3928, was established by researchers at the National Institute of Allergy and Infectious Diseases in the hopes that it will offer lasting security versus numerous influenza infection stress, consisting of those that may trigger a pandemic.
“This is an essential primary step on the multistep roadway to establishing a universal influenza vaccine,” institute Director Dr. Anthony Fauci. “We’re beginning with attempting to safeguard versus the group one influenza An infections.”
Fauci discussed that there have to do with 18 influenza An infections divided in between 2 groups, one and 2. A real “universal” vaccine needs to show efficiency versus all influenza infection stress, that will be accomplished just in progressive actions. The brand-new vaccine, then, is the initial step because it intends to successfully ward off a whole group of associated influenza viral stress.
The factor you require to get a brand-new influenza shot each year– and the vaccine itself should be upgraded each year– is since part of the infection continuously alters, Fauci stated. This phenomenon is called “antigenic drift.” To avoid antigenic drift, the brand-new vaccine prospect targets a more continuous part of the influenza stress, an element of the infection that differs reasonably little from stress to stress.
This very first stage of the medical trial, a human experiment, is being carried out at the National Institutes of Health Clinical Center in Bethesda, Maryland. The objectives of this primary step are restricted, Fauci stated: “You wish to see if the vaccine is safe– which it likely will be– and you wish to see if it causes the sort of reaction that would work.”
The experiment, led by Dr. Grace Chen of the institute’s Vaccine Research Center Clinical Trials Program, will need a minimum of 53 healthy grownups as much as 70 years of ages. Individuals will return for up to 11 follow-up gos to over a year to 15 months once they are offered the influenza shot. Individuals will not be exposed to a real influenza infection as part of the experiment since the researchers are checking for proof of an immune reaction in addition to security and negative effects.
Fauci anticipates “the typical kind of adverse effects” as individuals obtain from the present influenza vaccine, such as an aching arm, and does not anticipate any uncommon responses. The researchers want to start reporting lead to early 2020.
“If this works, then you transfer to Phase 2 and after that Phase 3,” Fauci stated, discussing that those are more involved human experiments taking a look at dosing and real-world efficiency. “I’m positive since we’re at the primary step, and lastly, we’re taking a look at something that’s genuinely created to be a universal influenza vaccine.”
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health group.
Schaffner stated that “getting something like a universal vaccine, which would safeguard versus an entire range of various stress, would be a significant medical advance.” The research study is “well-considered” and “now, we need to see if it works– how well it works.”
Flu impacts the whole world’s population, Schaffner stated. “If we had actually a considerably enhanced vaccine, we might do a dreadful great deal of excellent,” he stated. “We have high hopes.”
Read more: https://www.cnn.com/2019/04/05/health/flu-numbers-universal-vaccine-cdc-nih/index.html





